PHENOTYPIC PLATFORMS ARE TAKING OVER DRUG DISCOVERY

Quietly happening beneath the radar, phenotypic platforms are becoming integrated into drug discovery processes. Why this is happening and how are phenotypic assays transforming the stages of drug discovery?
Multiple drivers are prompting increased use of phenotypic platforms in drug discovery. The persistence of poor success rates is pushing program leaders to look for novel solutions to stubborn challenges. High numbers of programs continue to fail in clinical testing due to lack of efficacy and unexpected toxicities. Researchers are looking for new methods that might predict these problems ahead of time. Another driver is the ever growing availability of new assays and platforms with potential for addressing these problems. Progress in cell culture methods to improve access to human cell types along with advances in engineering techniques and detection technologies are providing new opportunities. Thirdly, as improvements in manufacturing methods and scale of phenotypic platforms are made, they become more affordable. Platforms that are affordable and can be run in high throughput mode are easier to validate (see here for why). Validation is important to build confidence in new methods for decision-making. Finally, enablements around data analytics, arguably in nascent stages, are helping drive the use of these platforms (see an example case study here). Tools that facilitate communication and interpretation of phenotypic assay results help program leaders more easily incorporate these data into program decisions.
So where are phenotypic platforms impacting drug discovery?
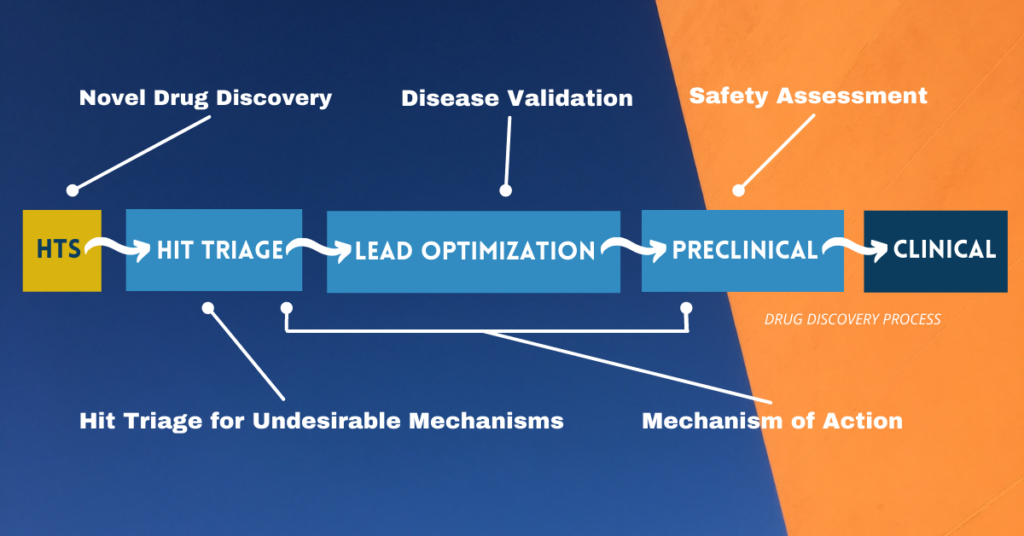
Graphic by the author adapted from Berg, 2021.
Most researchers are familiar with the application of phenotypic assays for primary screening, phenotypic drug discovery (PDD). PDD has been in the spotlight for some time (see Moffat et al., 2017; Vincent et al., 2020; Haasen et al., 2017; and the entire March 2021 issue of Cell Chemical Biology, including a personal perspective). New methods using CRISPR-Cas9 to develop genetically modified human primary cells represent a key advance driving new interest in high throughput phenotypic screens.
Phenotypic assays are also being applied for hit triage and prioritization (Vincent et al., 2020; Berg, 2020). We have proposed the application of specific phenotypic assays relevant to vascular and immune cell biology for use in early discovery to triage hits for undesirable pharmacology. These assays are recommended for both target-based and phenotypic discovery programs and identify compound activities that are problematic for interpreting eventual animal studies.
Mechanism of action is another area where phenotypic assays are making an impact. A number of phenotypic platforms are being applied for understanding mechanism of action, particularly for identifying unexpected off-targets. These include high content (Bray et al., 2016; Wardwell-Swanson et al., 2018), transcriptomics (Subramanian et al., 2017) and protein-based signatures (Berg, 2017).
For disease validation, we are seeing more advanced platforms such as tissue chips and organoids being applied for compound validation for efficacy. These platforms model complex tissue biology that is more representative of human disease. Example include systems using organoids (Hou et al., 2018; Yang et al., 2020; and Corrò et al., 2020), organs-on-a-chip (Jain et al., 2018; Tang et al., 2020), and human primary cell-based disease models (Berg et al, 2014).
Finally, phenotypic assays are increasingly being applied for detecting potential toxicity hazards. These include cardiomyocyte-based systems to detect arrhythmia and other cardiovascular system toxicities, and hepatocyte-based systems to explore liver toxicity mechanisms (Baudy et al., 2019; Müller et al., 2019; and Beilmann et al., 2019). Broad profiling using phenotypic platforms is also showing potential for assessing certain toxicity-related mechanisms.
While there is so much promise in these platforms, the growing number and diversity of potential assays raises practical concerns. Our challenge for each stage of the discovery process will be to identify and prioritize those assays that are most efficient and effective for the tasks at hand. It will be our mission to find the stars that shine most brightly.